Introduction: Venetoclax has demonstrated deep responses and sustained progression-free survival and is well tolerated in patients (pts) with previously untreated or relapsed/refractory (R/R) chronic lymphocytic leukemia/small lymphocytic leukemia (CLL/SLL) in clinical trials. Some early studies provided initial insight of venetoclax utilization in the real-world (RW), but RW evidence focusing on venetoclax effectiveness and safety is still limited. This study assessed venetoclax effectiveness, safety, and treatment patterns in CLL pts treated in clinical practice.
Methods: The CLL Collaborative Study of Real-World Evidence (CORE), a large multicenter chart review across 21 institutions in 4 countries, enrolled adult pts who initiated a first line (1L) therapy on/after 01/01/2012 or a new line of therapy (LOT) for R/R CLL/SLL on/after 02/12/2014 (current data cut through 06/08/2020). Clinical responses were abstracted from the medical records as assessed by treating physicians or investigators (iwCLL criteria were provided as reference). Overall response rate (ORR) was calculated as the proportion of pts with complete response (CR) or partial response (PR) out of pts with documented response assessments. Treatment patterns included choice of regimens and sequences, dose interruption, dose reduction, and discontinuation. Treatment discontinuation was defined as ending therapy for any reason(s) other than the completion of planned duration of therapy. Interruption was defined as a gap in the same LOT. All analyses were descriptive.
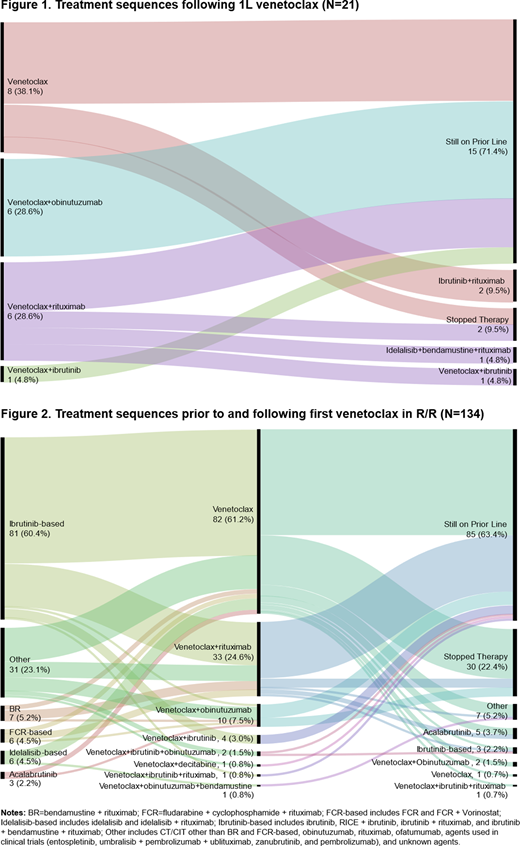
Results: Of the 1231 CLL pts in the CORE data, 155 received venetoclax (21 in 1L and 134 in R/R) and were included in this study. Most of the sample were male (65%) and the median age at venetoclax initiation was 67.5 (range 37-91). For high-risk features among pts with available data, 33% (43/132 pts) had del(17p) or TP53 mutation, 24% (31/131 pts) had complex karyotype (≥ 3 abnormalities), and 77% (61/79 pts) had unmutated IGHV. At venetoclax initiation, 46%, 40%, and 14% of 151 pts with available data had low, medium, and high tumor burden, respectively. Median follow-up time from venetoclax initiation across all lines was 7.0 (0.1-43.1) months. Median number of prior LOT for R/R pts was 2 (1-5); the largest proportion of pts (36%) received venetoclax in 2L followed by 3L (26%). In 1L, venetoclax was most frequently used (62%) in combination (Figure 1), while in R/R setting venetoclax monotherapy was more commonly used (61%) followed by venetoclax + rituximab (25%; Figure 2). Majority of R/R pts (63%) received Bruton's tyrosine kinase inhibitors (BTKi) prior to venetoclax (Figure 2).
Response was assessed and documented for 114 pts (74%). Among these, ORR was 75% (CR=40%, PR=35%) overall. Pts ≥65 years old had an ORR of 79% (CR=41%, PR=38%, n=74), and pts <65 years old had an ORR of 70% (CR=40%, PR=30%, n=40). ORR was 77% (CR=40%, PR=37%; n=30) for pts with del(17p) or TP53 mutation and 78% (CR=34%, PR=44%; n=64) for pts without del(17p) or TP53 mutation. ORR was 87% (CR=48%, PR=39%; n=23) for pts with complex karyotype and 74% (CR=32%, PR=42%; n=71) for pts without complex karyotype. ORR was 72% (CR=36%, PR=36%; n=53) for pts with unmutated IGHV and 91% (CR=36%, PR=55%; n=11) for pts with mutated IGHV.
Overall, 48% of pts had adverse events (AEs) recorded. Five R/R pts (3.2%) had TLS events (defined by Howard or Cairo-Bishop criteria), of which 3 (1.9%) were clinical. Of the 5 pts, 2 had high tumor burden, and 3 had medium burden. Other AEs of interest include neutropenia (17%), thrombocytopenia (9%), and diarrhea/colitis (5%).
Overall, 32% of pts discontinued venetoclax; 13% of all pts discontinued venetoclax due to AEs (primarily neutropenia [n=2] and thrombocytopenia [n=2]), followed by disease progression (8%). Venetoclax interruption rate was 14%, and dose reduction rate was 22% overall.
Conclusions: Consistent with trial experiences, venetoclax demonstrates high response rates, including high-risk groups, and a manageable safety profile with low rates of TLS in clinical practice. Despite limitations of RW research, this study provides useful insights into treatment practices with venetoclax. Future research with longer follow-up is warranted to better understand the long-term clinical outcomes associated with venetoclax regimens.
Zheng:AbbVie: Current Employment. Sail:AbbVie Inc.: Current Employment, Current equity holder in publicly-traded company. Roeker:Abbott Laboratories: Other: spouse with minority ownership interest ; American Society of Hematology: Research Funding; AbbVie: Other: spouse with minority ownership interest . Manzoor:AbbVie: Current equity holder in publicly-traded company. Tuncer:AbbVie: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees. Allan:Abbvie, Janssen, AstraZeneca, Pharmacyclics: Honoraria; Celgene, Genentech, Janssen, TG Therapeutics: Research Funding; Acerta, Genentech, Abbvie, Sunesis, Ascentage, Pharmacyclics, Janssen, AstraZeneca, BeiGene: Consultancy. Ujjani:Gilead/Kite: Consultancy, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Genentech: Consultancy, Honoraria; MorphoSys: Consultancy; Atara: Consultancy, Honoraria; Verastem Oncology: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Research Funding. Barr:Genentech: Consultancy; Merck: Consultancy; Abbvie/Pharmacyclics: Consultancy, Research Funding; Janssen: Consultancy; Celgene: Consultancy; Seattle Genetics: Consultancy; Verastem: Consultancy; TG therapeutics: Consultancy, Research Funding; Morphosys: Consultancy; Gilead: Consultancy; AstraZeneca: Consultancy, Research Funding. Brown:Novartis: Consultancy; Nextcea: Consultancy; Octapharma: Consultancy; MEI Pharma: Consultancy; Eli Lilly and Company: Consultancy; Astra-Zeneca: Consultancy; TG Therapeutics: Consultancy; Sunesis: Consultancy; Loxo: Consultancy, Research Funding; Sun: Research Funding; Rigel Pharmaceuticals: Consultancy; Pfizer: Consultancy; Catapult: Consultancy; Dynamo Therapeutics: Consultancy; Pharmacyclics: Consultancy; Morphosys: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other; Gilead: Consultancy, Research Funding; Genentech: Consultancy; BeiGene: Consultancy; Invectys: Membership on an entity's Board of Directors or advisory committees, Other: DSMC; Janssen: Honoraria; AbbVie: Consultancy; Kite: Consultancy; Juno/Celgene: Consultancy; Verastem: Consultancy, Research Funding; Acerta: Consultancy. Eyre:AbbVie: Consultancy, Honoraria, Other: travel support; KITE, AZ, Loxo Oncology at Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Other: travel support; Gilead: Consultancy, Honoraria, Other: travel support. Skarbnik:Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CLL Society: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Alexion: Consultancy; Beigene: Speakers Bureau; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Speakers Bureau; Verastem: Speakers Bureau; Pharmacyclics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Bannerji:Regeneron Pharmaceuticals: Research Funding; F. Hoffmann-La Roche Ltd/Genentech, Inc and Pharmacyclics LLC, an AbbVie Company: Research Funding; Sanofi-Pasteur: Other: Spouse is employee; AbbVie: Research Funding. Eichhorst:BeiGene: Consultancy, Honoraria, Other: travel support, Research Funding; AbbVie: Consultancy, Honoraria, Other: travel support, Research Funding; Janssen-Cilag: Consultancy, Honoraria, Other: travel support, Research Funding; F. Hoffmann-LaRoche: Consultancy, Honoraria, Other: travel support, Research Funding; ArQule: Consultancy, Honoraria, Other: travel support, Research Funding; Gilead: Consultancy, Honoraria, Other: travel support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: travel support, Research Funding; Oxford Biomedica: Consultancy, Honoraria, Other: travel support, Research Funding; Novartis: Consultancy, Honoraria, Other: travel support, Research Funding; Celgene: Consultancy, Honoraria, Other: travel support, Research Funding. Brander:Verastem: Consultancy, Honoraria, Other, Research Funding; NCCN: Other; Genentech: Consultancy, Honoraria, Other, Research Funding; Juno/Celgene/BMS: Other, Research Funding; MEI Pharma: Other, Research Funding; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Other, Research Funding; Pfizer: Consultancy, Other; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; NCCN: Other; Novartis: Consultancy, Other; Teva: Consultancy, Honoraria; Tolero: Research Funding; Ascentage: Other, Research Funding; ArQule: Consultancy, Other, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Tolero: Research Funding; Teva: Consultancy, Honoraria; Novartis: Consultancy, Other; DTRM: Other, Research Funding; AstraZeneca: Consultancy, Honoraria, Other, Research Funding; BeiGene: Other, Research Funding. Sharmokh:AbbVie: Current Employment, Current equity holder in publicly-traded company. Jiang:AbbVie: Current Employment, Other: may hold stock or options. Pena:AbbVie: Current Employment, Current equity holder in publicly-traded company. Kamalakar:AbbVie: Current Employment, Other: may hold stock or other options. Emechebe:AbbVie: Current Employment, Current equity holder in publicly-traded company. Pivneva:Novartis: Consultancy, Other: Irina Pivneva is an employee of Analysis Group, Inc which received consultancy fees from Novartis.. Burne:Analysis Group, Inc., which has received consultancy fees from AbbVie: Current Employment. Guerin:Abbvie: Consultancy, Other; Novartis Pharmaceuticals Corporation: Consultancy, Other: Annie Guerin is an employee of Analysis Group, Inc. which received consultancy fees from Novartis.; Sanofi Genzyme: Consultancy, Other: Annie Guerin is an employee of Analysis Group, Inc. which received consultancy fees from Sanofi Genzyme.. Davids:Celgene: Consultancy; Research to Practice: Honoraria; AbbVie: Consultancy; Sunesis: Consultancy; Genentech: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; MEI Pharma: Consultancy, Research Funding; Surface Oncology: Research Funding; BeiGene: Consultancy; Novartis: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Bristol Myers Squibb: Research Funding; Janssen: Consultancy; Adaptive Biotechnologies: Consultancy; Ascentage Pharma: Consultancy, Research Funding; Gilead Sciences: Consultancy; Eli Lilly: Consultancy; Syros Pharmaceuticals: Consultancy; Zentalis: Consultancy; Merck: Consultancy; TG Therapeutics: Consultancy, Research Funding; Verastem: Consultancy, Research Funding. Mato:AbbVie: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; Loxo: Consultancy, Research Funding; Janssen: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal